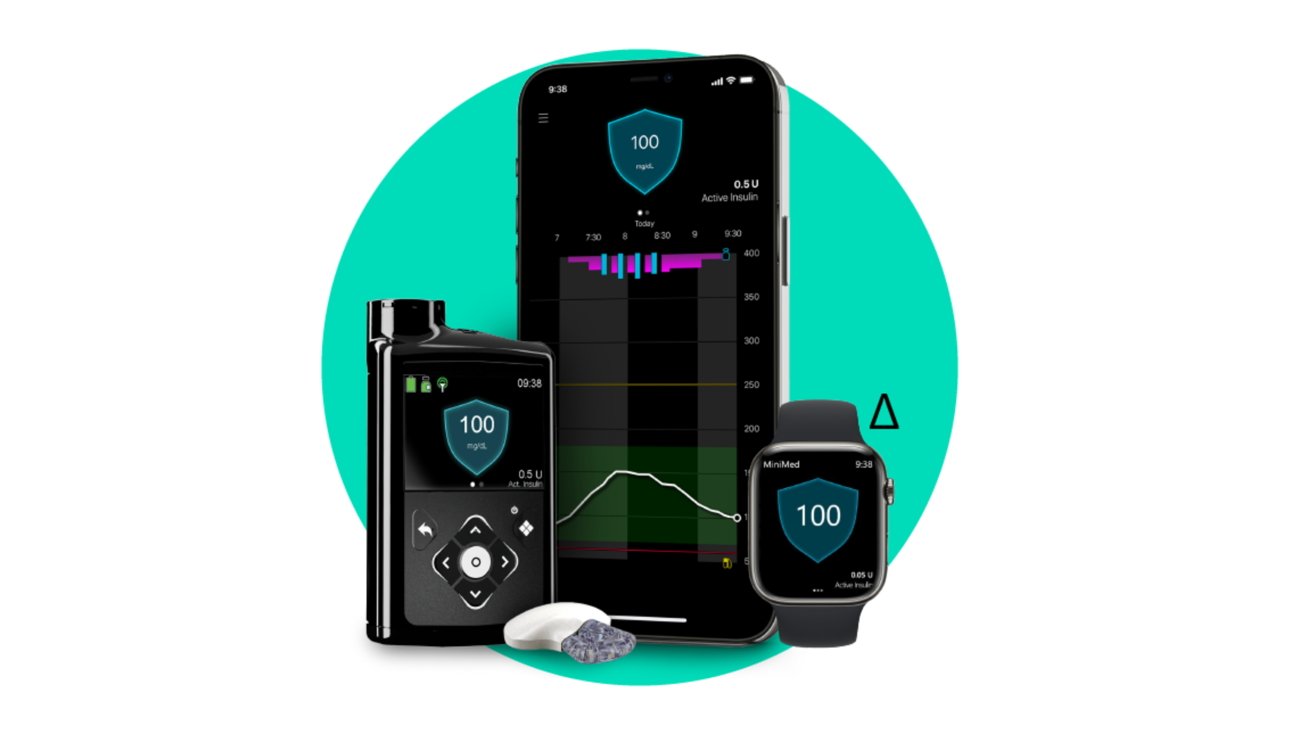
Medtronic has introduced that its long-awaited 780G diabetic remedy system has gained FDA approval which incorporates help for iPhone and Apple Watch monitoring.
The Medtronic 780G system encompasses the insulin remedy pump and the Guardian 4 fixed glucose monitor — or CGM. Mixed, Medtronic is ready to create a hybrid closed-loop supply system that may problem automated corrections primarily based on blood sugar.
It additionally works to make corrections with automated meal corrections. Customers can enter their estimated carbs consumed and if the pump detects your blood sugar spiking quickly, it is going to problem a stronger correction dosage to convey you inside vary.
If you happen to forgot to bolus for a meal, it too will ship a correction bolus to counteract the carbs. Must you drift low, insulin supply shall be suspended.
Like with Dexcom, excessive and low blood sugar will present in your Apple Watch and iPhone with new readings showing each 5 minutes. In contrast to Tandem’s system, you’re unable to manage the insulin pump out of your cellphone at the moment.